Ernest Rutherford Atomic Theory: Ernest Rutherford celebrated as the father of nuclear physics, earned this title through his groundbreaking work on radioactivity and atomic structure. Born in New Zealand, Rutherford’s journey from a small town to becoming one of the most influential scientists of the 20th century fascinates and inspires.
Who is Ernest Rutherford
Ernest Rutherford was born on August 30, 1871, near Nelson, New Zealand. He was the fourth of twelve children in a hardworking family. His father, James Rutherford, was a farmer, and his mother, Martha Thompson, was a schoolteacher. Interestingly, when he was born, his first name was mistakenly registered as “Earnest.”
Rutherford’s early education took place at Havelock School, and later he attended Nelson College. His academic talents were evident early on, and he won a scholarship to study at Canterbury College, University of New Zealand. Here, he excelled in mathematics and physics, earning his Master’s degree with first-class honors in 1893. During his time at university, he also invented a new type of radio receiver.
 A Promising Scientist
A Promising Scientist
In 1895, Rutherford received a prestigious Research Fellowship from the Royal Commission for the Exhibition of 1851, which allowed him to study at the University of Cambridge. Under the guidance of J.J. Thomson at the Cavendish Laboratory, Rutherford began his groundbreaking work on radio waves. He even briefly held the world record for detecting electromagnetic waves over the greatest distance.
Ernest Rutherford What Did He Discover
Rutherford’s scientific achievements began to pile up quickly. In 1898, he moved to Canada to become the chair of physics at McGill University in Montreal. There, he discovered that radioactivity involved the spontaneous disintegration of atoms, which was a revolutionary idea at the time. Before this discovery, atoms were considered indestructible.
Ernest Rutherford alpha and beta rays
He also coined the terms “alpha” and “beta” rays to describe two types of radioactive emissions. Later, he introduced the concept of half-life, showing that radioactive materials decay at a predictable rate. His work on the transformation of elements was published in the “Law of Radioactive Change” in 1903, laying the foundation for modern nuclear physics.
Ernest Rutherford Awards
In 1908, Rutherford was awarded the Nobel Prize in Chemistry for his research into the disintegration of elements and the chemistry of radioactive substances. However, his work didn’t stop there. During World War I, he worked on a top-secret project to develop submarine detection methods using sonar.
Rutherford also played a significant role in advancing science in his home country. In 1926, his efforts led to the creation of the Department of Scientific and Industrial Research (DSIR) in New Zealand, which supported education and research.
The Rutherford Atomic Model
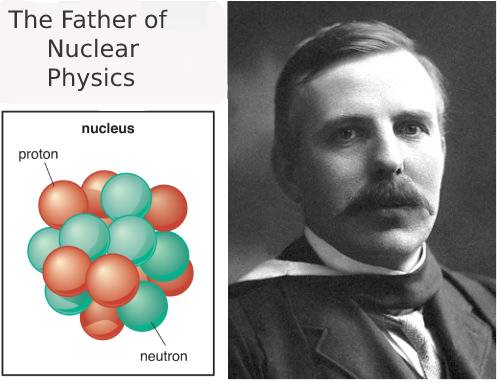
One of Rutherford’s most significant contributions was his model of the atom. Through his famous gold foil experiment, he demonstrated that atoms are mostly empty space with a small, dense nucleus at the center. This model revolutionized our understanding of atomic structure and is still fundamental to physics today.
Ernest Rutherford Gold Foil Experiment
In 1909, Ernest Rutherford conducted a famous experiment that changed how we think about atoms. He wanted to understand what atoms are made of, so he and his team shot tiny, positively charged particles (called alpha particles) at a thin sheet of gold foil. They expected these particles to go straight through the foil with only small changes in direction.
Plum Pudding Model
At that time, scientists believed in the “plum pudding model,” which imagined the atom as a spread-out cloud of positive charge with electrons scattered throughout. They thought the particles would pass through without much trouble. But the results were surprising. Most particles did go through the foil, but some bounced off at sharp angles, and a few even bounced straight back. This was unexpected and showed that the old model was wrong.
Ernest Rutherford Experiment
Rutherford concluded that the atom has a tiny, dense, positively charged center, which he called the nucleus. The electrons, he suggested, orbit this nucleus, and most of the atom is empty space. This discovery completely changed our understanding of the atom and led to the Rutherford model, which became the basis for modern nuclear physics.
Later Life and Legacy
In his later years, Rutherford continued to contribute to science and received numerous awards and honors. He served as President of the Royal Society from 1925 to 1930 and was knighted as Baron Rutherford of Nelson in 1931. In 1933, he received the T.K. Sidey Medal from the Royal Society of New Zealand for outstanding scientific research.
Ernest Rutherford’s Birth and Death
Ernest Rutherford was born on August 30, 1871, near Nelson, He passed away on October 19, 1937, at the age of 66. In the years leading up to his death, Rutherford had been suffering from a small hernia, which he had neglected. Despite undergoing an emergency operation just days before, complications arose, leading to what doctors described as “intestinal paralysis.”New Zealand. He was given the high honor of being buried in Westminster Abbey, near other great British scientists like Isaac Newton.
Ernest Rutherford Fun Facts
- His first name was mistakenly registered as “Earnest.”
- The element “Rutherfordium” is named in his honor.
- He appears on the New Zealand $100 note.
- Two of his brothers tragically drowned in 1886.
The Father of Nuclear Physics
Ernest Rutherford’s work forever changed our understanding of the atomic world. His discoveries laid the groundwork for nuclear physics, and his legacy continues to influence science today. From a small-town boy in New Zealand to a global scientific icon, Rutherford’s life is a testament to curiosity, hard work, and the power of scientific inquiry.
Ernest Rutherford’s Contributions to Science
What did Ernest Rutherford discover?
Ernest Rutherford is the founder of atomic nuclei, and protons, and made significant contributions to the understanding of radioactivity. His gold foil experiment led to the realization that atoms consist of a small, dense nucleus surrounded by space, fundamentally altering the atomic model.
Which activity best demonstrates Ernest Rutherford’s creativity?
Rutherford’s creativity is best demonstrated by his gold foil experiment. By directing alpha particles at a thin sheet of gold foil, he was able to challenge and ultimately disprove the existing “plum pudding model” of the atom, leading to the discovery of the atomic nucleus.
What did Ernest Rutherford’s gold foil experiment demonstrate about an atom?
The gold foil experiment demonstrated that atoms have a small, dense, positively charged nucleus at their center. It also showed that most of the atom is empty space, as the majority of alpha particles passed through the foil with little deflection.
What did Ernest Rutherford expect to happen when he aimed a beam of particles at a thin gold foil?
Rutherford expected that the alpha particles would pass through the gold foil with minimal deflection, in line with the “plum pudding model” of the atom, which suggested that the positive charge was diffused throughout the atom.
What did Ernest Rutherford’s gold foil experiment demonstrate about atoms?
The experiment demonstrated that atoms are mostly empty space with a small, dense nucleus. This finding contradicted the previously accepted “plum pudding model,” which assumed the positive charge was spread out within the atom.
Which statement best summarizes the importance of Ernest Rutherford’s gold foil experiment?
The gold foil experiment was crucial because it disproved the “plum pudding model” and provided the first evidence of the atomic nucleus, fundamentally changing our understanding of the atomic structure and leading to the development of the modern atomic model.
Which describes Ernest Rutherford’s experiment?
Rutherford’s gold foil experiment involved directing a beam of alpha particles at a thin sheet of gold foil and observing how the particles scattered. This experiment revealed that atoms have a tiny, dense nucleus and that most of the atom is empty space, challenging the existing atomic model at the time.
When did Ernest Rutherford make his discovery?
Ernest Rutherford made his groundbreaking discovery of the atomic nucleus in 1909, during his gold foil experiment. This experiment was pivotal in the development of nuclear physics.
How did Ernest Rutherford contribute to the atomic theory?
Ernest Rutherford contributed to atomic theory by discovering the atomic nucleus and proposing a new model of an atom. This model laid the groundwork for modern atomic theory.
What did Ernest Rutherford contribute to the atomic theory?
Rutherford’s most significant contribution to atomic theory was the discovery of the nucleus, leading to the Rutherford atomic model. This model proposed that the atom consists of a central nucleus surrounded by electrons, overturning the earlier “plum pudding” model.